
Le programme a été élaboré par le Comité de planification du Congrès scientifique annuel du PRDTC, coprésidé par Mamatha Bhat (Thèmes 4 et 5) et Michael Khoury (Thèmes 1, 2, 3 et 5) avec l’appui des responsables des Thèmes et de la communauté du PRDTC. Les autres membres du comité de planification sont :
- Suze Berkhout (Thèmes 1, 2 et 5)
- Sean Delaney (Thème 1, patient partenaire)
- Caigan Du (Thèmes 2 et 3)
- Marie-Chantal Fortin (Thèmes 1, 2, 3, 4 et 5)
- Francis Migneault (Thème 3)
- Javairia Rahim (Thème 4, stagiaire)
- Matthew Weiss (Thèmes 1 et 2)
Le Congrès scientifique annuel 2022 du PRDTC s’est déroulé sous forme d’événement hybride, avec la possibilité d’y assister en personne ou virtuellement. La santé et le bien-être des participants à la réunion est demeurée notre principale priorité. Nous nous sommes engagés à fournir un environnement sûr, productif et accueillant pour tous les participants. Veuillez consulter notre politique de santé et sécurité entourant la COVID-19 ici.
PRÉ-CONGRÈS SCIENTIFIQUE
Mardi 6 décembre 2022
L’horaire est présenté à l’heure normale du Pacifique (HNP)
Activités sociales

Participants virtuels, rejoignez-nous pour une session de réseautage en ligne afin de faire connaissance et d’échanger avec d’autres membres de la communauté du PRDTC ! Cette session sera animée par Patricia Gongal, directrice générale du PRDTC ainsi que Sean Delaney, patient partenaire.
- Date : Mardi 6 décembre
- Heure : 10h00 HP | 13h00 HE
- En virtuel seulement
CONGRÈS SCIENTIFIQUE ANNUEL DU PRDTC
Mercredi 7 décembre 2022
L’horaire est présenté à l’heure normale du Pacifique (HNP)
An overview of the CDTRP highlights of the year 2022 (30 minutes presentation + 15 min Q&A)
Speakers
This session is kindly sponsored by Astellas Pharma, Inc., Paladin Labs Inc. and Takeda Canada.

International Forum – Turning Recommendations into Reality (45 minutes)
Speakers
Moderator
Description
In 2021, Transplant Québec and the Canadian Donation and Transplantation Research Program co-hosted The International Donation and Transplantation Legislative and Policy Forum (the Forum). The Forum assembled 57 international experts in donation and transplantation, including patient, family, and donor partners, to provide guidance on the structure of an ideal organ and tissue donation and transplantation (OTDT) system.
Seven domain working groups were responsible for generating the topics that merit recommendations, gathering and summarizing relevant evidence related to these topics, and creating recommendations. The recommendations link evidence and ethical concepts to legislative and policy reform and are applicable to jurisdictions developing or reforming their OTDT system.
🤸🏃🕺 Stretch your legs and join us for a movement break with Manoela de Paula Ferreira! 🤸🏃🕺
Focus on Donation
Moderator
Rethinking policies of mandated anonymity in deceased organ donation (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstracts
Historically, all Canadian provinces had policies or legislation barring organ donation organizations from disclosing information identifying deceased donors’ families and organ recipients, even with their mutual consent. The rationale was to protect stakeholders from such risks as financial exploitation, unreasonable or unwelcome emotional demands, feelings of indebtedness, and complicated grief. Stakeholders have long expressed frustration with this restrictive regime, believing that they ought to be permitted to reap the benefits that research has shown can arise when the two sides meet. With the rise of social media, deceased donors’ families and organ recipients are taking matters into their own hands by seeking to independently identify and contact the people on the other side of their donation story. When they do, they may benefit – but they are also unwittingly exposing themselves to the potential harms and complications described above, with no resources in place to support them. The irony in places like Ontario is that the very legislation designed to protect stakeholders is now a barrier to organ donation organizations developing policies that can support and protect stakeholders who encounter one another on platforms like Facebook. The rise of social media is gradually making restrictive policies obsolete. It is time for policymakers to face up to the realities of the age of social media. Restrictive regimes will no longer work. Policies should be developed to respond to this challenge by allowing identity disclosure in those instances where both sides agree. Whatever the ultimate parameters of less restrictive policies, a balanced approach to donor family and recipient contact will help to protect anonymity for those who want it and responsibly facilitate contact for those who do not.
ISODP Journal Watch (15 minutes presentation)
Speakers
Description
The International Society for Organ Donation and Procurement (ISODP) is the organization advancing donation through science, developing professionals and inspiring networks supporting organ donation to improve organ transplantation worldwide. The ISODP Journal Watch is an educational resource and benefit to the ISODP members. “Two of the primary goals for the Society are to enhance resources to improve donation practices and to establish an integrated network of donation professionals.”
The ISODP Journal Watch started in 2021 with a team of UK Researchers. The 2022 edition has been be led by a Canadian team, Drs Matt Weiss and Sonny Dhanani, with the help of the Canadian Donation and Transplantation Research Program (CDTRP) Trainees and Management Team to identify and summarize relevant articles on organ donation.
The ultimate goal of Journal Watch would be to lead to the creation of a journal entirely dedicated to organ donation.
Pragmatic and Adaptive Trials in ODT (40 minutes presentations + 20 min Q&A)
Moderators
The need for pragmatic and adaptive trials in ODT (5 minutes)
- Speaker: Marat Slessarev
Case study: SIGNET RCT (lessons learned, barriers & facilitators, emerging partners, next steps) (10 minutes)
- Speaker: Dan Harvey
Towards pragmatic and platform trials in ODT (25 minutes)
- Speaker: Maureen Meade
🍽️🥣 🥗 This lunch is kindly sponsored by AstraZeneca 🍽️🥣 🥗

Focus on Ex situ/Ex vivo Perfusion
Moderators
Facilitators and Barriers of Ex Situ Heart Perfusion (ESHP) in Pediatric Donation (20 minutes presentation + 10 minutes Q&A)
Speaker
Abstract
Purpose: Despite medical advances, pediatric heart transplant (HTx) candidates are at significant risk for morbidity and mortality. Ex Situ Heart Perfusion (ESHP) is a method for continuous perfusion of the donor heart, allowing for extended out-of-body time and functional assessment of the organ. Currently available devices are targeted to adults and have been used in the context of neurological and circulatory determination of death. Pediatric-specific devices are in development, but before adoption, knowledge of stakeholder attitudes towards ESHP is needed. The aim of this study was to explore pediatric HTx stakeholders’ understanding and perspectives of ESHP.
Methods: A virtual focus group study was conducted international pediatric HTx stakeholders. Data was analyzed using qualitative content analysis.
Results: There were 17 participants from 12 institutions representing 3 countries. Findings were organized under the topics of: general knowledge, indications, risks, adoption/barriers, and pediatric-specific considerations. Knowledge ranged from limited grasp of the technology to first-hand experience in clinical practice. Discussions encircled the current use of ESHP in adult-sized HTx candidates, and potential future uses in infants and young children. In all focus groups, it was discussed that risks need to be considered regarding ESHP itself, including the potential of damaging the donor heart, subjecting recipients to unquantified risks, and potential adverse impacts on pediatric HTx programs if poor outcomes occur. Concerns surrounding the material costs and human resources associated with ESHP were also discussed. While all discussions highlighted the need for pediatric ESHP devices, participants emphasized the need for rigorous research and implementation oversight of ESHP to navigate ethical issues, program administration, and support best practices in the context of technological innovation.
Conclusion: For stakeholders to become adopters of ESHP, they must understand the goals, benefits, and risks. This project represents the first step in the adoption of new technology, and the knowledge gained will form the basis for future education, clinical trial design, and rollout of pediatric ESHP technologies.
24-hour negative pressure ventilation ex-situ lung perfusion with a porcine transplantation model (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Background: Lung transplantation has a wait-list mortality of 30% due to a shortage of high-quality donor lungs. Ex-situ lung perfusion (ESLP) allows for improved preservation and reconditioning of marginal quality donor lungs to expand the donor pool, increasing donor utilization rates in some centres by 20%. To date, the longest pre-clinical preservation period with favourable transplantation data is 24-hours using positive pressure ventilation ESLP. Negative pressure ventilation has been shown to result in reduced lung injury and inflammation, yet reliable 24-hour preservation has yet to be achieved.
Objectives: The aim of this study was to refine our protocol of negative pressure ventilation (NPV)-ESLP to achieve reliable 24-hour preservation and assessment with acceptable in-vivo transplantation data.
Methods: Twelve double-lung blocks from 45-55kg pigs were preserved on normothermic NPV-ESLP for 24-hours using a cellular solution with autologous packed red blood cells. Lung protective ventilation and perfusion strategies were employed throughout preservation. Six left lungs were transplanted into recipients post-ESLP and reperfused for 4-hours to evaluate the impact on in-vivo post-transplant lung function. Final assessment of the transplanted left lung was performed with the right lung clamped. Lung oxygenation capacity (PF ratio = PaO2/FiO2), dynamic lung compliance (Cdyn), pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), and lung weight-gain were monitored.
Results: All twelve lung blocks demonstrated stable lung function during 24-hours of NPV-ESLP. At 24-hours, PF ratios were 473.9 ± 29.5 mmHg, Cdyn was 20.2 ± 2.8 mL/cmH20, PAP was 15.0 ± 1.1 mmHg, PVR was 570.7 ± 46.9 dynes/s/cm5, percentage weight-gain after ESLP was 54.66 ± 8.0. Acceptable lung oxygenation was demonstrated in all six transplanted left lungs with a mean PF ratio >300 mmHg (329.5 ± 21.7), equivalent to primary graft dysfunction (PGD) grade 0. Isolated left lung weight gain (%) post-transplant was 15.3 ± 39.02.
Conclusions: Although limited by small sample size, this study illustrates that reliable 24-hours of normothermic NPV-ESLP with a cellular perfusate is achievable, resulting in acceptable post-transplant oxygenation capacity. Prolonged preservation will allow for the treatment more advanced lung pathology and the elimination of geographic barriers for transplant. Future studies will strive to achieve 36-hours of NPV-ESLP with favourable transplantation data.
Ninjurin1 Contributes to Lytic Cell Death in Hepatic Ischemia-Reperfusion Injury (20 minutes presentation + 10 minutes Q&A)
Speaker
Abstract
Background: Liver transplantation is the gold standard therapy for many terminal liver diseases but the field remains limited by the availability of donor organs. Ischemia reperfusion injury (IRI) is an inherent part of organ transplantation, and is particularly problematic when utilizing high-risk organs, a necessary step for expanding the donor pool. Mechanistically, IRI leads to hepatocellular damage, lytic cell death and allograft dysfunction. Recently, ninjurin1 (NINJ1) has been implicated in macrophages as the common executioner of multiple lytic cell death pathways seen in IRI. Here we investigate the contribution of NINJ1 to hepatic IRI, including its role in mediating lytic cell death in hepatocytes. We focus on the pyroptosis cell death pathway, as whether hepatocytes are capable of undergoing pyroptosis has been hotly debated
Methods: For in vivo liver IRI, mixed-sex cohorts of Ninj1 knockout or wildtype littermate control mice were subjected to segmental warm liver injury consisting of 1 hour of ischemia and 6 hours of reperfusion. Liver injury and inflammation were evaluated by histopathology, serum AST/ALT levels and flow cytometric analysis of inflammatory infiltrate. For in vitro experiments, primary mouse hepatocytes and Kupffer cells were stimulated to undergo lytic cell death in normoxic or hypoxic conditions and membrane rupture assayed by the release of lactate dehydrogenase.
Results: Our data demonstrate that, like macrophages and Kupffer cells, primary hepatocytes are capable of undergoing pyroptosis. Furthermore, membrane rupture across cell types is abrogated in Ninj1 knockout cells as compared to wildtype. In vivo, Ninj1 genetic deficiency reduces hepatic IRI as compared to wildtype littermate control animals.
Conclusion: This work demonstrates that hepatocytes can directly undergo pyroptosis. Furthermore, it offers the first evidence that targeting membrane disruption through NINJ1 inhibition reduces hepatic IRI in vivo, thereby demonstrating a new potential therapeutic target for prevention of allograft injury during liver transplantation.
Normothermic ex vivo kidney perfusion preserves ATP generation and graft function after warm ischemia, which is further enhanced with pharmacologic mitochondrial protection (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Background: Normothermic Ex-vivo kidney machine perfusion (NEVKP) is a novel preservation technique. We recently determined that NEVKP preserves the expression of proteins involved in mitochondrial biogenesis in kidneys. We hypothesize that ex vivo machine perfusion will replenish energy levels in mitochondria, thereby restoring mitochondrial function and reducing injury in kidney grafts. AP39, a mitochondria-targeted hydrogen sulfide donor, has been shown to stimulate mitochondrial electron transport and improve cellular bioenergetic function. Here, we investigated whether administering AP39 during NEVKP protects mitochondrial function and protects renal grafts from ischemia reperfusion injury.
Methods: Porcine kidneys were subjected to 60 minutes of warm ischemia (WI) followed by 5 hours of static cold storage (SCS) or NEVKP. The warm ischemia grafts were subsequently divided into three groups: SCS group, NEVKP group, and a group in which AP39 was additionally administered during NEVKP (NEVKP + AP39). After contralateral nephrectomy, grafts were auto-transplanted and animals were followed for 3 days. Renal function and ATP levels were assessed.
Results: All animals (n=5-6 in each group) survived the follow-up period. Grafts preserved with NEVKP had lower serum creatinine (SrCr) on postoperative day 3 compared to the SCS group. Treatment with NEVKP + AP39 further reduced SrCr when compared to the NEVKP group (SCS vs NEVKP vs NEVKP + AP39: 12.7±1.1 vs 7.6±2.8 vs 3.5±1.1 mg/dl, mean±SD). We measured ATP in biopsy-derived cell suspensions from grafts stored using SCS, NEVKP, and NEVKP + AP39. ATP levels were increased in the NEVKP group compared with SCS group at the time of pre-implantation and this level was further increased when AP39 was administered. (1.9±11.4 vs 73.6±9.3 vs 121.6±36.3 nM/10000cells).
Conclusions: For grafts subjected to warm ischemia, NEVKP has the potential to preserve ATP generation and graft function, which is further enhanced with mitochondria-targeted hydrogen sulfide donor, AP39.
Quantitation of mitochondrial damage-associated molecular patterns in perfusate and bile for analysis of donor liver quality during ex-vivo NMP (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Background: Tissue damage during liver transplantation (LT) results in expulsion of mitochondrial damage-associated molecular patterns (mitoDAMPs), including mitochondrial DNA (mtDNA), into the extracellular space. High mtDNA levels positively correlate with negative clinical outcomes, and mtDNA is emerging as a predictive biomarker for a variety of disease processes. Normothermic machine perfusion (NMP) has emerged as an attractive alternative to traditional static cold storage (SCS), yet there remains an unmet need for an agile and universal marker of graft function during NMP. We hypothesized that mtDNA could be quantitated in perfusate and bile collected during ex vivo NMP of donor livers prior to transplant and would correlate with other metrics of graft quality.
Methods: DNA was isolated from perfusate (n=29) and bile (n=18) collected from donor livers undergoing NMP. MtDNA levels were measured via quantitative polymerase chain reaction (qPCR) using primers specific to mtDNA gene targets (COXI, CytB, ND1, ND6) and standard curves generated by commercially available synthetic oligonucleotides of primer-specific amplicons.
Results: MtDNA can be quantitated in both perfusate and bile samples collected during NMP. MtDNA quantitation via qPCR with an amplicon-specific standard curve accurately reproduced values measured by droplet digital PCR (ddPCR). Concentration of mtDNA in perfusate was positively correlated with warm ischemia time (COXI, p=0.042; CytB, p=0.041) and donor lactate (CytB, p=0.012; ND1, p=0.040). Donor international normalized ratio (INR) showed significant positive association with mtDNA in both perfusate (CytB, p=0.003; ND1, p=0.048) and bile (CytB, p=0.048; ND1, p=0.049; ND6, p=0.032).
Conclusions: Here, we demonstrate the feasibility and reproducibility of mtDNA quantitation in both perfusate and bile via qPCR and show association with clinical parameters. We anticipate that mtDNA will serve as a stable and inexpensive marker for liver function during NMP, which may inform the expected course of disease and illuminate opportunities for precision medicine in patients undergoing LT.
The Frictions of Futurity and Cure in Transplant Medicine (“Frictions”) project team will host a 1-hour discussion lounge during the CDTRP 2022 Annual Scientific Meeting. The lounge is conceived to be a space to articulate diverse viewpoints, share knowledges, practices and ways of knowing, and engage with different forms of understanding the challenges, tensions, promises, and hopes that are attached to transplantation and in particular to the way that transplantation is both reveals and troubles concepts such as cure, risk, kin, and care. As social science and arts-based researchers interested both in understanding these aspects of transplantation, we would both be facilitating and documenting the conversations that take place.
What is the study about?
This study aims to better understand what it is like living with an organ transplant: from waiting to receive an organ, to challenges post-transplant. Investigators, who are artists and researchers, want to to understand how transplantation experiences impact individuals in multiple places and at different times. This study seeks to identify the different goals and needs of patients, their family members, physicians, and other experts involved in transplant. The results of this study will help contribute to best practices for care in solid-organ transplantation and to policies and guidelines on care.
Why are researchers doing direct observation?
Understanding the experiences and meanings of transplant recipients and clinical team members is central to this study. This information provides insights about care priorities, relationships, and emotion that may not arise in interviews. Direct observation enables the researcher to witness important interactions unfold in real-time, making for a more nuanced, sensitive, and accurate understanding of living with organ transplant.
In addition to direct observation, researchers are interested in interviewing participants and inviting participants to join a group art-making workshop and/or work with a researcher-artist to create an audio-visual account (Photovoice) of their transplant experience. These activities will take place at UHN or online.
The research team and artists involved in the project intend to claim sole ownership of any results that would come from this study. You will not receive any financial benefit that might come from the results of this study.
Contact
For questions about direct observation or if you would like to see the field notes taken during your visit, please contact:
Principal Investigator: Suze Berkhout | suze.berkhout@uhn.ca
Indigenous Book Club: Growing reconciliation in ODT through Indigenous literature
Host
The First Nations and Métis Organ Donation and Transplantation Network introduces its first book club. Participants will be asked to read the novel Five Little Indians by Michelle Good and attend a discussion gathering hosted by Dr. Caroline Tait, research lead of the Network. The book club is hoping to attract medical professionals who work in organ donation and transplantation, specifically liver transplant. The purpose of the book club is to provide a safe space for discussion about inter-generational colonial impacts upon Indigenous peoples, specifically the impact of residential schools, and how processes of colonialism directly contribute to health and social inequities experienced by Indigenous peoples today. The goal of this initial Book Club is to draw upon Indigenous fiction to create a safe and productive space to discuss complex health issues impacting First Nations, Métis, and Inuit peoples.
Requirement: Each participant must read the novel in advance and bring the book with them to book club.
PRE REGISTRATION REQUIRED – SPACE LIMITED
Trainee Three Minute Thesis Presentations (Themes 3-4)
Join the next generation of donation and transplantation researchers as CDTRP trainees, and other graduate students across Canada present their research as a Three Minute Thesis (3MT). 3MTs allow students to foster effective presentation and communication skills as they explain their work in 3 minutes and in 3 slides. Presentations during this session are related to CDTRP’s Theme 3 (Engineer and Allocate Better Grafts) and Theme 4 (Tailor an Optimal Immune System for Each Patient).
As 3MT presentations are designed for non-specialist audiences, YOU will have the opportunity to evaluate each presentation LIVE. The top presenter, as selected by the CDTRP members in the audience, will be awarded a $250 prize.
SEPARATE REGISTRATION REQUIRED HERE.

VOTE HERE
(1) Aisha Adil
- Ex Vivo Perfusion De- and Recellularization of Rat Hindlimbs for Vascular Composite Allotransplantation
- Details
(2) Jorge Castillo
- Developing an Ex-vivo organ perfusion system to model kidney disease
- Details
(3) Hyunyun Kim
- Autophagy Inhibition Aggravates Ischemia-Reperfusion Injury-Induced Microvascular Injury
- Details
(4) Christine Wardell
- The trogocytosis assay: A blood-based assay to detect alloreactive immune cells
- Details
(5) Marwa Sadat
- Normothermic ex vivo perfusion of the murine pancreas to model type 1 diabetes
- Details
(6) Samrat Ray
- Expanding the donor pool using Normothermic Ex vivo Machine Perfusion model in pancreas transplantation
- Details
(7) Imane Kaci
- Apoptotic exosome-like vesicles mediate immune response dysregulation and kidney dysfunction following ischemia-reperfusion injury
- Details
10-MINUTE BREAK
(8) Ruixuan (Sunnie) Yang
- Gram-Positive and Gram-Negative Bacterial Surface Constituents Hinder Kidney Cell Viability In Vitro
- Details
(9) Maude Lanoie
- Apoptotic exosome-like vesicles transfer bioactive mRNA to endothelial cells via phosphatidylserine-dependent macropinocytosis
- Details
(10) Rohit Malyala
- Determination of blood pressure targets for goal-directed anesthesia in renal transplant
- Details
(11) Emmanuel Nogueira
- Use of Pancaspase inhibitor during Normothermic ex vivo liver perfusion: a strategy to reduce ischemia reperfusion injury in pig liver grafts
- Details
(12) Khairunnadiya (Nadia) Prayitno
- Transcriptomic Changes in Liver Transplant Recipients with Non-Alcoholic Steatohepatitis Indicate Dysregulation of Wound Healing
- Details
(13) Gopika Punchhi
- Deep Learning to Predict Trajectories and Identify Features Associated with Death and Transplant in Waitlisted NASH Patients
- Details
Writing grants and papers with style: how to win friends and influence people
Hosts
To convince reviewers, win grants, and be cited, it is critical that others are able to understand your work and its importance. Clear, well-structured writing is the best way to make that happen. This workshop will overview principles and practical techniques for writing effective scientific documents, including manuscripts and grant proposals. We will discuss common problems with scientific texts, techniques to improve structure and clarity, and other tips to make your reader’s life easier. The workshop will cover:
- Why scientific texts can be difficult to read
- Overview of effective structure at the document, paragraph, and sentence level
- Barriers to clear communication of your ideas
- Diagnosing problems in your writing and troubleshooting what you find
Open to all trainees and dinner will be provided. You MUST send a writing sample to info@cdtrp.ca by Monday, December 5 in order to attend the Workshop.
Jeudi 8 décembre 2022
Pragmatic and Adaptive Trials in ODT Workshop
Aim: to initiate develop of a framework for conducting international pragmatic and adaptive trials in ODT
SEPARATE REGISTRATION REQUIRED HERE.
Focus on Exercise in Transplantation (30 minutes présentations + 15 min Q&A)
Moderators
The MedBIKE: Evaluating a Novel Telemedicine and Video Game-Linked Exercise Platform for Pediatric Heart Transplant Recipients – 10 minutes presentation
Speaker
Abstract
Children who are heart transplant recipients (HTRs) have reduced fitness levels compared with the general population. Exercise programs in childhood HTRs, however, have not been well studied. Our group has developed a home-based, video-game linked exercise bike that allows for a live video-feed along with heart tracing, and oxygen saturation monitoring (the MedBIKETM). We have established a MedBIKETM program for children born with severe forms of heart disease. We are now seeking to study this in childhood HTRs.
To do this, we will include 10-18 year old HTRs followed at the Stollery Children’s Hospital. Participants will undergo a baseline assessment of their fitness and physical activity levels, and questionnaires will be provided to evaluate their attitudes towards physical activity and their health-related quality of life. Participants will then be randomly assigned to a MedBIKETM or regular care (control) group. The MedBIKETM group will begin a 12-week home-based high-intensity interval training (HIIT) program. Following the program, a follow-up assessment (identical to the baseline assessment) will be performed for the MedBIKETM and control group participants. The groups will then switch, so that the control group starts the MedBIKETM program and the original MedBIKETM group returns to usual care. A second follow-up assessment will then be performed for all participants at the completion of the second 12-weeks.
It is our hope that the findings from this innovative study will provide important information to help us build upon our work, in our pursuit to support active, healthy living in pediatric HTRs.
Acceptability and feasibility of the Kidney Transplant Physical Activity and Social Club (KEeP ACTIVe Club) – 10 minutes presentation
Speaker
Abstract
Background: We developed the “Kidney Transplant Physical Activity and Social Club” (KEeP ACTIVe Club) to offer support for kidney transplant recipients (KTRs) to improve their levels of physical activity (PA) and knowledge in cardiovascular disease, and to break the isolation and loneliness they may feel after transplantation. The main objective was to assess the acceptability and feasibility of the KEeP ACTIVe Club. We also evaluated pre-post intervention changes in self-efficacy for PA, levels of PA and social support and personal relationships.
Methods: The KEeP ACTIVe Club lasted six months and offered one educational session about benefits of PA and risk factors for cardiovascular disease, online social networking (via a Facebook closed group led by two patient partners), peer and professional mentorship, and a menu of options for PA. Acceptability and feasibility were measured by the proportion of KTRs approached who participated and the number who completed the intervention. Self-efficacy was measured using a multidimensional self-efficacy scale for exercise, the level of PA was assessed by the the International Physical Activity Questionnaire (IPAQ), and social support was measured by the MOS Social Support Survey.
Results: One hundred twenty-one KTRs were approached over 15 months (43 initially interested and eligible; 78 refused); 18 (15%) participated in the study (mean age 50.5 ±16.1; 50% female; 7 months – 24 years post-transplant). Forty-four percent of participants chose to do virtual exercises classes led by a kinesiologist and several walked with the patient partner. Eleven KTRs (61% of enrolled) finished the post-intervention questionnaires. Self-efficacy improved by 7.7 points (±16.0), the IPAQ improved by 2,273 MET minutes/week (±4,450) and social support remained stable at 3.5 points.
Conclusions: The KEeP ACTIVe Club is feasible. Acceptability was low (possibly influenced by the pandemic), however, self-efficacy for PA and level of PA improved in those who participated in the intervention.
Feasibility of a Home-Based Exercise Program in Lung and Liver Transplant Recipients for Management of Post-Transplant Metabolic Syndrome: A Pilot Randomized Controlled Trial – 10 minutes presentation
Speaker
Abstract
BACKGROUND/RATIONALE: Post-transplant metabolic syndrome (PTMS) is prevalent across solid organ transplant recipients (25-50%) at 1-year. PTMS (comprised of impaired glucose tolerance, obesity, hypercholesterolemia and hypertension) has been associated with increased risk for hospital re-admissions, cardiovascular morbidity and decreased survival. In the first-year post-transplant, facility based training has been beneficial in improving health-related quality of life (HRQL) and physical function, but it remains unclear whether home-based exercise training is feasible and can be prescribed with appropriate intensity to have effects on PTMS beyond the first-year.
OBJECTIVES: 1) To evaluate the feasibility of a 12-week individualized, home-based exercise program in LTx and OLT recipients 2) To assess estimates of intervention efficacy on PTMS risk factors, exercise self-efficacy, and HRQL.
METHODS: Randomized control trial (RCT) of 20 LTx and 20 OLT recipients with ≥ 2 cardio-metabolic risk factors at 12-18 months post-transplantation randomized to home-based exercise training (intervention) versus usual care. The intervention arm will have weekly supervision by an exercise professional to assist with progression of aerobic and strength training and support exercise self-efficacy. Nutritional counselling will be provided to both groups. Feasibility will be evaluated through recruitment rate, adherence, safety, and participation satisfaction. Secondary outcomes will be ascertained at baseline and 12 weeks, including PTMS factors, HRQL, and exercise self-efficacy.
RESULTS: Enrollment began in July 2021 with 83 LTx or OLT recipients approached in clinic with ≥ 2 PTMS risk factors as of June 30, 2022. 39/83 (47%) were interested in the study, whereas 44 (53%) declined due to time constraints or technological difficulties. 16/39 (41%) met inclusion criteria with 11 participants randomized. Top reasons for exclusion were medical illness, too active, or no internet availability. There was no loss to follow-up or safety concerns. Enrollment is planned until March 31, 2023.
CONCLUSIONS: This ongoing RCT highlights some unique aspects of recruitment in the post-transplant period in recipients with PTMS. A home-based exercise program may be an important post-transplant intervention for improving PTMS, but education on importance of exercise training is needed.
Bringing Narrative and Visual Research Methods to Transplant: An Experiential Workshop
Speakers
Description
The purpose of this activity is to experience one way that researchers use artistic or creative visual methods to explore peoples’ experiences. On a blank page, using whatever materials you have handy or that you are interested in, make a scribble. Don’t think about what you are doing or drawing, just make marks on a page. Let your hands move freely. You can modify the page (crumpling it, tearing it) if you like. Use different colours, different markers, crayons, other pencils or pens, You can make patterns or different marks with varying thickness. Move into a non-verbal space by just letting yourself try out the materials in a free-form way. When we’re done, we’ll use the scribbles to introduce ourselves and the facilitators will model the kind of discussion that might follow from an art activity in a research setting.
🤸🏃🕺 Stretch your legs and join us for a movement break with Manoela de Paula Ferreira! 🤸🏃🕺
Focus on Equity, Utility and Access
Moderator
Exploring the information needs of African, Caribbean, and Black Community Members Regarding Living Donor Kidney Transplantation in Toronto, Ontario (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Background: In Canada, patients with kidney failure from African, Caribbean, and Black (ACB) communities are 60-70% less likely to receive a living donor kidney transplant (LDKT) compared to White patients. U.S. research suggests that providing transplant-related information may improve equitable access to LDKT. However, little is known about the information needs of ACB communities in Canada related to this topic. In this qualitative study, we aimed to better understand the LDKT-related information needs of ACB communities in Toronto, ON.
Methods: Purposive and snowball sampling were used to recruit self-identified ACB participants for in-person and virtual focus groups (FGs) (January-November 2020). In collaboration with community partners, an exploratory qualitative approach was used to guide discussions on racial and ethnic identity, and knowledge and perspectives on LDKT. FGs were audio recorded and transcribed verbatim. Thematic analysis directed the development of codes and themes and were co-generated by the research team. Analysis was informed by the tenets of Critical Race theory, which prioritizes racism as a determinant of health.
Results: Of the 81 participants, 48% self-identified as Caribbean, 36% as North American Black/African, 4% as Central/West African, and 6% as North African. One major theme that emerged was a desire for tailored and trustworthy information on LDKT. Participants expressed mistrust in the health care system, rooted in historic and current experiences of anti-Black racism, impacting their willingness to consider the information on LDKT that is typically provided by medical institutions. This may be one reason why many study participants described being unaware of the causes, consequences, and treatment options for kidney failure. Participants emphasized that future development of resources must incorporate important cultural, preventive, and holistic elements.
Conclusion: Future information on LDKT provided to ACB communities would require collaboration with community partners to ensure that it is tailored and trustworthy.
Examining questions of equity and utility for Indigenous patients living with end-stage organ failure (1-hour panel discussion)
Speakers
Moderator
Description
Indigenous peoples in Canada face a higher disease burden leading to end-stage organ failure, and face both geographic and systemic barriers to accessing organ donation and transplantation (ODT). Despite the inequities that exist, Indigenous healthcare leaders remain on the margins of decision-making about ODT healthcare services, policies and legislation. This panel, made up of two-Indigenous patient/family partners, one Indigenous healthcare leader, and one researcher, merges the lived experiences of patients with a broader sociological analysis of the equity issues described in the patient/family narratives. The objective of the panel is to bring the lived experience of Indigenous peoples to a national forum and ground this experience in a learning context that looks beyond calls for cultural safety to address systemic challenges faced by Indigenous patients and families.
Xenotransplantation: The Path to Clinical Application

Dr. Pierson holds the W. Gerald and Patricia Austen Chair in Cardiac Surgery and is the Scientific Director of the Center for Transplantation Sciences at Massachusetts General Hospital, Boston, MA, USA. He is a Professor of Surgery at Harvard Medical School, and visiting surgeon at MGH, where he participates clinically in thoracic transplantation and cardiac surgical intensive care. He is an established NIH-funded investigator in the areas of translational cardiac allograft tolerance induction and the immunobiology of lung, heart, kidney, and liver xenotransplantation, and author of over 175 peer-reviewed original scientific papers, review articles, and book chapters.
Dr. Pierson received his medical degree from the Columbia University, and trained at University of Michigan, Massachusetts General Hospital, and Papworth Hospital, Cambridgeshire, England, in affiliation with Cambridge University. He has served on the faculty at Vanderbilt University (1994-2002), University of Maryland (2002-2018), and Harvard University (2018-present).
Dr. Pierson is board certified in general and thoracic surgery, and is a fellow of the American College of Surgeons. He is a member of the American Surgical Association, American Association of Thoracic Surgeons, American Society of Transplant Surgeons, International Society of Heart and Lung Transplantation, Society of Thoracic Surgeons, and Society of University Surgeons, among others. He is a past president of the International Xenotransplantation Association (2007-09), and current IXA Ethics Committee Chair. He co-chaired WHO’s 1st International Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials, held in Changsha, PRC, and was raporteur for the Geneva follow-up meeting.
Focus on Immunology
Moderators
Glycocalyx engineering of vascular endothelium to achieve localized immunomodulation (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Introduction: The glycocalyx (eGcx), made up of membrane-bound glycoproteins and proteoglycans, is of particular interest due to its ability to control cell to cell communication and the activation of immune response through cell recognition. During organ transplantation, inflammation and oxidative damage occurs and leads to the shedding and damage of the eGcx layer; this has been linked to organ failure and rejection. We developed an enzymatic approach to modify the surface of the endothelium with immunosuppressive polymers ex vivo to recapitulate the eGcx to achieve localized immunomodulation and prevent rejection post-transplantation.
Methods & Results: We developed a method using tissue transglutaminase (tTGase) as the surface immobilizing enzyme and polyglycerol polymers containing sialic acid or sulfate moieties that is compatible with organ storage conditions (in UW at 4°C). In vitro mechanistic studies were performed using EaHy.926 cells to replicate the endothelium and PBMCs and CAR-T cells were used to test immune cytotoxicity. Modified endothelial cells were able to evade CAR-T cell induced cytotoxicity and reduced TNF release in M1 macrophages. In vivo efficacy of graft rejection was assessed through aortic vessel or renal grafts from BALB/c donor mice into C57BL/6 recipient mice. Modified vessel allografts showed reduced medial thickening, leukocyte infiltration and donor-specific antibodies. Lastly, modified renal allografts revealed less leukocyte infiltration and mesangial expansion after 30 days with healthy renal function.
Conclusion: Here, the use of a polymer-mediated organ engineering approach leads to vascular protection that prevents immune-mediated rejection of organ transplants. Ex-vivo delivery of these immune cloaking polymers that engineer the blood vessel lumen allow for localized immune protection, making this an enticing and viable strategy for the reduction of broad-active immunosuppressants post-transplantation.
The role of sex and T cells in natural vs induced ABO antibody production in mice (10 minutes presentation + 5 minutes Q&A)
Speaker
Abstract
Introduction: Interaction of ‘natural’ ABO antibodies (nAbs) with their cognate AB(H)-antigens (Ags) poses a high risk of rapid rejection of ABO-incompatible (ABOi) organ transplants. We previously demonstrated that a clear understanding of factors influencing ABO nAbs is crucial for successful ABOi heart transplantation. Here we investigated anti-A nAbs vs. intentionally-induced Abs (iAbs) with regard to role of sex and T cell requirement.
Methods: Adult wild-type (WT) and CD4 T cell knock-out (CD4KO) mice (C57BL/6 (B6) background) received weekly i.p. injection x3 of human ABO-A blood cell membranes (Hu-A BCM; 100ul of 10% v/v) or left untreated. Serum anti-A Ab was measured by hemagglutination assay using ABO-A erythrocytes from our A-transgenic mouse line. To test for T cell help and/or suppression, sex-matched CD4+ T cells (8-12×106/mouse) or CD4+CD25+ T cells (1.7-2.8×106/mouse) from spleens of WT mice were transferred to CD4KO mice. After adoptive transfer, CD4+ T cell reconstitution in peripheral blood was confirmed and mice were left untreated or challenged with Hu-A BCM and assessed for anti-A Ab.
Results: In contrast to WT mice, untreated CD4KO females produced dramatically more anti-A than males, rising substantially with puberty, and this was significantly suppressed in both sexes by adoptive transfer of sex-matched CD4+ T cells. Unlike WT mice, attempted sensitization of CD4KO mice with Hu-A BCM failed to induce additional anti-A beyond the already high levels in either sex; CD4+ T cell adoptive transfer rendered CD4KO mice responsive to A-sensitization. CD4+CD25+ T cell transfer into CD4KO mice neither suppressed anti-A nAbs nor rendered them responsive to A-sensitization (Figure).
Conclusions: When ABO ‘natural’ antibodies are discriminated from intentionally induced Abs, several important findings emerge: 1) Anti-A nAbs are produced without CD4+ T cell help in a sex- and age-dependent manner, suggestive of a role for sex hormones in regulating anti-A nAbs. 2) CD4+ T cells, but not CD4+CD25+ regulatory T cells, down-regulate anti-A nAb production. 3) In contrast to anti-A nAbs, production of anti-A iAbs was CD4+ T cell-dependent without a sex bias.
Preliminary results of the first clinical trial to prevent graft rejection in heart transplant children employing a cellular therapy with autologous Treg obtained from thymic tissue (thyTreg) – 20 minutes presentation + 10 minutes Q&A
Speaker
Abstract
Due to their suppressive capacity, regulatory T cell (Treg) therapy is a very promising alternative to prevent rejection and achieve indefinite graft survival. Clinical trials conducted in adults with peripheral blood Treg demonstrate the safety of the therapy, although its efficacy seems to be limited. To overcome these limitations, we have developed a novel GMP-compatible protocol to obtain Treg from thymuses routinely discarded during pediatric cardiac surgeries (thyTreg).
After receiving the approval by the Spanish Drug Agency (AEMPS), we have started the first worldwide phase I/IIa clinical trial (NCT04924491) that evaluates the autologous use of thyTreg in preventing rejection in children undergoing heart transplantation. A single dose of fresh thyTreg is administered to the patient intravenously at day +8±2 post-transplant and the remaining thyTreg are cryopreserved.
To date, 4 patients have received a thyTreg dose. The four thyTreg products infused showed very high viability (>94%) and purity (CD25+FOXP3+) ≥85% in all cases. The procedure’s safety is confirmed, and the preliminary results obtained in the children treated suggest that with a single thyTreg infusion it is possible to maintain the reserve of Treg cells, even in these patients who have undergone thymectomy and treated with immunosuppressive therapy. So far, we have yet recruited six more patients in the trial who are still on the heart transplant waiting list, completing the 10 patients planned for this clinical trial.
ThyTreg cell constitutes a new therapeutic strategy to induce graft tolerance that could establish a new paradigm in the context of solid organ transplantation.
The Frictions of Futurity and Cure in Transplant Medicine (“Frictions”) project team will host a 1-hour discussion lounge during the CDTRP 2022 Annual Scientific Meeting. The lounge is conceived to be a space to articulate diverse viewpoints, share knowledges, practices and ways of knowing, and engage with different forms of understanding the challenges, tensions, promises, and hopes that are attached to transplantation and in particular to the way that transplantation is both reveals and troubles concepts such as cure, risk, kin, and care. As social science and arts-based researchers interested both in understanding these aspects of transplantation, we would both be facilitating and documenting the conversations that take place.
What is the study about?
This study aims to better understand what it is like living with an organ transplant: from waiting to receive an organ, to challenges post-transplant. Investigators, who are artists and researchers, want to to understand how transplantation experiences impact individuals in multiple places and at different times. This study seeks to identify the different goals and needs of patients, their family members, physicians, and other experts involved in transplant. The results of this study will help contribute to best practices for care in solid-organ transplantation and to policies and guidelines on care.
Why are researchers doing direct observation?
Understanding the experiences and meanings of transplant recipients and clinical team members is central to this study. This information provides insights about care priorities, relationships, and emotion that may not arise in interviews. Direct observation enables the researcher to witness important interactions unfold in real-time, making for a more nuanced, sensitive, and accurate understanding of living with organ transplant.
In addition to direct observation, researchers are interested in interviewing participants and inviting participants to join a group art-making workshop and/or work with a researcher-artist to create an audio-visual account (Photovoice) of their transplant experience. These activities will take place at UHN or online.
The research team and artists involved in the project intend to claim sole ownership of any results that would come from this study. You will not receive any financial benefit that might come from the results of this study.
Contact
For questions about direct observation or if you would like to see the field notes taken during your visit, please contact:
Principal Investigator: Suze Berkhout | suze.berkhout@uhn.ca
15:30 - 17:00 | Developing a Framework for Evaluating Patient, Family, and Donor Impact within CDTRP
Developing a Framework for Evaluating Patient, Family, and Donor Impact within CDTRP
Speaker
Description
CDTRP launched the Patient, Family, and Donor (PFD) Partners Platform in 2016 to integrate patients voices within it’s research structure. Since then, PFD partners have brought their unique and experiential knowledge to CDTRP with an expectation that the network’s activities are relevant and reflective of the PFD community. Has CDTRP met these expectations? This session brings together PFD partners and patient engagement experts to discuss how we can evaluate PFD impact within CDTRP. We will (1) discuss why evaluating the role of patient, family, and donor partners within research networks is important and outline the roadmap to evaluate PFD involvement. (2) In working groups, we will co-develop a list of evaluation priorities for CDTRP. CDTRP PFD Theme 5 Co-Lead, Sandra Holdsworth, initiated this session with an abstract submission for the Annual Scientific Meeting. Your input throughout this session will help set the foundation for our evaluation.
SEPARATE REGISTRATION REQUIRED HERE.
The Frictions of Futurity and Cure in Transplant Medicine (“Frictions”) project team will host a 1-hour discussion lounge during the CDTRP 2022 Annual Scientific Meeting. The lounge is conceived to be a space to articulate diverse viewpoints, share knowledges, practices and ways of knowing, and engage with different forms of understanding the challenges, tensions, promises, and hopes that are attached to transplantation and in particular to the way that transplantation is both reveals and troubles concepts such as cure, risk, kin, and care. As social science and arts-based researchers interested both in understanding these aspects of transplantation, we would both be facilitating and documenting the conversations that take place.
What is the study about?
This study aims to better understand what it is like living with an organ transplant: from waiting to receive an organ, to challenges post-transplant. Investigators, who are artists and researchers, want to to understand how transplantation experiences impact individuals in multiple places and at different times. This study seeks to identify the different goals and needs of patients, their family members, physicians, and other experts involved in transplant. The results of this study will help contribute to best practices for care in solid-organ transplantation and to policies and guidelines on care.
Why are researchers doing direct observation?
Understanding the experiences and meanings of transplant recipients and clinical team members is central to this study. This information provides insights about care priorities, relationships, and emotion that may not arise in interviews. Direct observation enables the researcher to witness important interactions unfold in real-time, making for a more nuanced, sensitive, and accurate understanding of living with organ transplant.
In addition to direct observation, researchers are interested in interviewing participants and inviting participants to join a group art-making workshop and/or work with a researcher-artist to create an audio-visual account (Photovoice) of their transplant experience. These activities will take place at UHN or online.
The research team and artists involved in the project intend to claim sole ownership of any results that would come from this study. You will not receive any financial benefit that might come from the results of this study.
Contact
For questions about direct observation or if you would like to see the field notes taken during your visit, please contact:
Principal Investigator: Suze Berkhout | suze.berkhout@uhn.ca
Trainee Three Minute Thesis Presentations (Themes 1-2-5)
Join the next generation of donation and transplantation researchers as CDTRP trainees, and other graduate students across Canada present their research as a Three Minute Thesis (3MT). 3MTs allow students to foster effective presentation and communication skills as they explain their work in 3 minutes and in 3 slides. Presentations during this session are related to CDTRP’s Theme 1 (Improve a Culture of Donation), Theme 2 (Inform Universal Practices for Donation), and Theme 5 (Restoring Long-Term Health).
As 3MT presentations are designed for non-specialist audiences, YOU will have the opportunity to evaluate each presentation LIVE. The top presenter, as selected by the CDTRP members in the audience, will be awarded a $250 prize.
SEPARATE REGISTRATION REQUIRED HERE

VOTE HERE
(1) Alexandra Frankel
- Embodied Survivorship: Using Arts-Based Methods to Complicate Biomedical Narratives of Organ Transplantation and Improve Care
- Details
(2) Wajiha Ghazi
- Physical function impairment among patients on dialysis and kidney transplant recipients
- Details
(3) Manoela Ferreira, Marie-Hélène Normand & Sandrine Juillard
- Effects of telerehabilitation on physical function, mental health and health-related quality of life in solid organ transplant recipients A systematic review
- Details
(4) Amy Thachil
- Investigating serum immunometabolomic profiles associated with pediatric kidney transplant alloimmune outcomes
- Details
(5) Amal Trigui
- Longitudinal evolution of nutritional risk and muscle function of patients waiting for liver transplantation and the effect of early nutritional intervention following the transplantation
- Details
(6) Chloe Wong-Mersereau
- Expanding Temporalities: Complicating Psychosocial Tools for Liver Transplant Survivors through Critical Discourse Analysis and Digital Storytelling
- Details
(7) Ke Fan Bei, Princess Okoh, and Aisha Adil
- Evaluation of indications for genetic assessment in living kidney transplant donors and relevant Canadian practices in light of the current international guidelines
- Details
10-MINUTE BREAK
(8) David Zorko
- Autoresuscitation After Circulatory Arrest: An Updated Systematic Review
- Details
(9) Farnaz Farahbakhsh
- Iranian Donors Save Lives Campaign to Engage Iranian Peoples as Stem Cell Donors
- Details
(10) Ruo Hao Zhang
- The Lay of the Land in Qualitative Liver Transplant Research: A Scoping Review and Network Mapping Study
- Details
(11) Sibele Schuantes
- Time elapsed between cells, tissues and organs donation and transplantation and adverse events detection
- Details
(12) Amina Silva
- Organ Donation Following Medical Assistance in Dying: A Scoping Review
- Details
(13) Cindy Wen
- “Losing face” as a potential barrier to living donor kidney transplantation for Chinese Canadians in Toronto, Ontario: Preliminary findings from a qualitative analysis
- Details
(14) Katina Zheng
- Live donor liver transplantation in primary sclerosing cholangitis: an indicator of an organ allocation system not addressing patient need
- Details
On site. More details to come.
Vendredi 9 décembre 2022
PART 1 – 1 hour
A Vision for the Future of the Canadian Donation and Transplantation Research Ecosystem and the role of CDTRP
Moderator
Panelists
Description
There is consensus that the lack of a unified approach to Canadian donation and transplantation research and innovation represents a major barrier to establishing pan-system priorities for research and to maximizing the benefits of research. Driven by the Organ Donation and Transplantation Collaborative, funded by Health Canada, and supported by the Canadian Donation and Transplantation Research Program, the project “Mapping the Canadian Research and Innovation Ecosystem in Organ Donation and Transplantation” sought to create a clear view of the research and innovation ecosystem and create a shared vision for the future. Who are all of the stakeholders in the ecosystem and what roles do they play? What are the current gaps and opportunities for improvement in research and innovation in Canada? What is the future vision for the research and innovation ecosystem in Canada? What are the next steps in bringing this vision closer to reality?
This session will overview and discuss the national, community-generated vision for a more effective and aligned research and innovation ecosystem and what CDTRP can bring to the table.
PART 2 – 45 minutes
CDTRP Media Workshop Series on ODT & Health Literacy
Moderator
Panelists
Description
In April 2021, CDTRP launched its Media Workshop Series on Organ Donation and Transplantation (ODT) & Health Literacy. This Workshop series aims to bring together journalists and media specialists (traditional and social) with researchers, health professionals, and patient, family and donor partners with expertise in the field of donation and transplantation and engage conversations for the goal of making it easier for accurate information and meaningful stories of public interest to reach Canadians.
Through the first Workshop entitled « Learning from each other« , we exposed the two sides of the coin: a first point of view from the media/journalistic side and a second from the ODT side. We then engaged a conversation with a various panel of researchers, journalists, health care professionals and patient partners. More details here.
In collaboration with the Health Law Institute from the University of Alberta, the second Workshop, entitled “Engaging the public online during policy change” speakers from different backgrounds were asked to share their recent and extensive experience engaging the public online. They discussed the complexities around hosting and moderating discussions, creating accurate informative content, and responding to misinformation. More details here.
Now, we are engaging organizations in the ODT community who engage with media in a discussion with an eye to greater collaboration – how can we better work together to build a strong culture of organ and tissue donation in Canada? In this 45-min panel discussion, we will discuss the missions and roles of key or in the donation and transplantation community, programs they have in place for the dissemination of information to the public and media, strengths and limitations as organizations, and how we could all work hand in hand in a more collaborative fashion.
Addressing critical emerging issues in COVID-19 in transplant recipients
Moderator
Speakers
Description
Transplant recipients remain at high risk of severe COVID-19 outcomes. For transplant recipients surviving COVID-19 and/or experiencing the extreme stress associated with the pandemic, little is known about effective strategies to support recovery of quality of life. Studying these questions in Canada is challenging, given the pace of variant evolution and drug development, as well as regional variations in therapeutic practices and public health policies.
This session will provide an overview of how the CDTRP aims to leverage our pan-Canadian research network to create an agile, national framework that can address urgent and emerging issues, incorporating priorities of clinicians, researchers, and patients/families. Our adaptive design will assess the safety, efficacy, and cost-effectiveness of therapies in transplant patients, considering short and long-term clinical outcomes, and other outcomes critical to patients and their families, including mental health, life participation, and financial burden. This project will provide the actionable knowledge needed by policymakers, for clinical guidelines, and by transplant patients and their families.
This session is kindly sponsored by AstraZeneca and GSK.

Artificial Intelligence and Transplantation (40 minutes presentations + 20 min Q&A)
Moderators
Introduction Remarks (10 minutes presentation)
Speaker
Machine Learning Models to Predict Long-term Allograft Survival in Liver Transplant Recipients (10 minutes presentation)
Speaker
Abstract
Background: Liver allograft failure arises in around 25% of liver transplant (LT) recipients, resulting in death or need for retransplantation. Currently, there are no standard clinical tools or methodology for assessing a given patient risks of graft failure. Providing an accurate and interpretable method for individualized risk estimation of graft failure is essential.
Methods: Using longitudinal patient follow-up data, we develop and validate our models on 82,959 liver transplant recipients from the Scientific Registry of Transplant Recipients. We identified six clinical biomarkers that are most predictive from a time-varying Cox regression model: albumin, bilirubin, creatine, INR, AST, and ALT. We further compared such an interpretable model against four other complex models including random survival forest, DeepSurv, survival transformer and a recurrent neural network. We assessed the model performance by time-dependent concordant index and the average of this metric over time.
Results: The random forest model achieves the best average concordance over time horizons of 0.828 ± 0.008 (95% CI) on a separate test dataset, while the simplest and interpretable Cox model remains competitive with a concordance of 0.821 ± 0.007 (95% CI). The recurrent neural network has the least concordance of 0.816 ± 0.007 (95% CI).
Conclusion: Different data-driven risk scoring models for continuous monitoring of liver transplant recipients demonstrated compelling results in assessing the dynamic risk of graft failure over time post-transplant. They provide additional risk measures based on massive data analysis to aid the decision-making process for possible preventive treatment; we believe this digital health tool could be widely applicable for monitoring graft functioning during follow-ups to enhance the long-term outcomes of liver transplant recipients. Future work includes external validation of model performance on Canadian datasets.
DynaMELD: Accurate, Equitable Modeling of End-Stage Liver Disease (10 minutes presentation)
Speaker
Abstract
Background: The current MELD Na-based prioritization system results in disadvantage to specific patient subgroups, including women and those with cholestatic liver disease. We present DynaMELD, a deep learning-based model that achieves estimation of risk superior to the MELD-Na, and reduces sex-based disparities in prioritization.
Methods: Models were trained on data from 121,647 waitlisted patients in the Scientific Registry of Transplant Recipients. Each patient record contained 330 features collected at time of listing, 11 features collected at each check-in prior to transplant, and time of event/censorship. We compare performance of our DynaMELD model, a neural network trained on the aforementioned 342 features, against benchmarks of the MELD, MELD-Na, MELD 3.0, and Cox regression.
Results: DynaMELD obtained a significantly higher concordance index (0.862 (0.851-0.873)) than the benchmark models (0.836 (0.825-0.848), 0.833 (0.8217-0.845), 0.840 (0.829-0.851), and 0.850 (0.839-0.860), respectively). Additionally, in a 5-year simulation (2016-2021) of 19,070 patients using the LivSim simulator, allocating livers according to the DynaMELD risk score resulted in 192 fewer instances of pre-transplant mortality than the MELD-Na (a 1% reduction). Additionally, whereas the MELD-Na yielded a 0.9% higher rate of pre-transplant mortality in women, DynaMELD admitted only a 0.4% higher rate of pre-transplant mortality in women in simulation; 96 fewer women died under the DynaMELD policy.
Conclusion: Our results suggest that leveraging nonlinear risk models on longitudinal data, and incorporating additional patient covariates (including rates of change), provide superior estimates of relative mortality risk to the MELD-Na, the existing clinical standard of care. We find that these models may better serve disadvantaged subpopulations of patients, such as women. We propose that such work has the potential to improve and render more equitable the allocation of deceased donor livers.
Ethical Consideration commentary (10 minutes)
Speaker
Activités sociales en présentiel
Wine Tour
Two options are available for a vineyard tour of the beautiful Okanagan Valley:
- Tuesday, December 6 from 3:00 to 6:00 pm
- Friday, December 9 from 3:00 to 6:00 pm
The cost per person is $170, which includes the tour, transportation, and tastings. Please note that space is limited to 14 people per tour, and we need a minimum of eight to run a tour. Spaces will be allocated first come, first serve.
If you would like to participate please contact info@cdtrp.ca.
« Heading to a winery is a wonderful way to support local, and get into the spirit with your friends and family, and to pick up wines to enjoy over the holidays! » Sneak peak here.


Souper de début des célébrations du 10e anniversaire du PRDTC
Joignez-vous à nous pour un souper gastronomique dans la magnifique salle Okanagan. C’est une excellente occasion d’échanger avec vos collègues dans un environnement détendu.
- Date : Jeudi 8 décembre
- Heure : 19h00
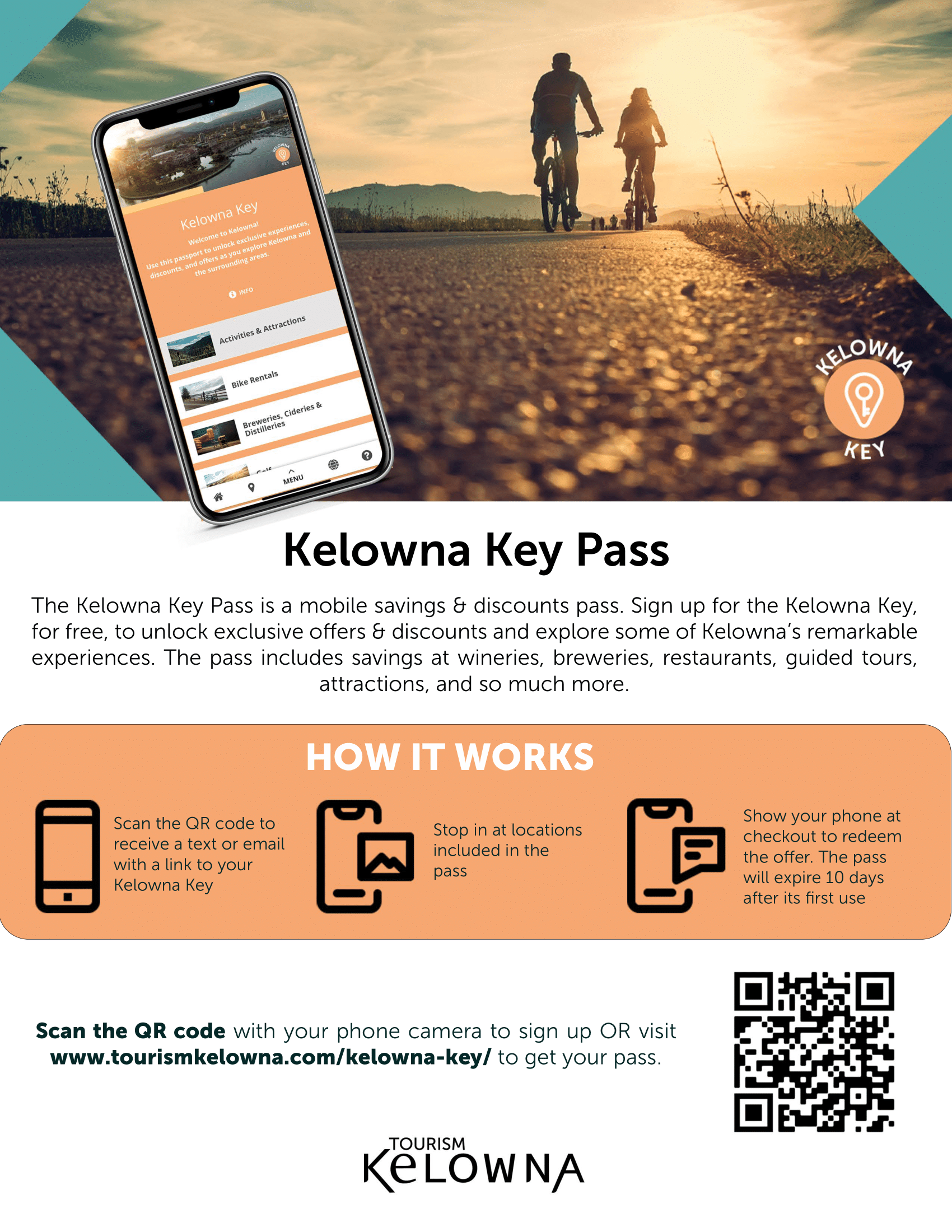
Kelowna Key Pass
Make the most of your time here by signing up to the Kelowna Key and unlock exclusive experiences, discounts, and offers throughout the city. You can use the pass to explore award winning wineries and micro-breweries, tour vineyards, visit farms and orchards, rent bikes, choose from a great selection of dining options, and so much more.
The Kelowna Key is a free passport filled with offers and discounts exclusive for conference and event delegates. Over 60 businesses can be found on the pass including wineries, restaurants, guided tours, and attractions.
Sign up for free, then just show the pass at participating businesses to redeem the offer.
We hope this pass encourages your guests to explore some of the remarkable experiences Kelowna and area have to offer!
*Some businesses may require a reservation, please check offers terms and conditions.










 ity of Alberta and has received training in patient-oriented research through the University of Calgary’s PACER (Patient and Community Engagement Research) Program. He is the Patient, Family, and Donor Partnerships & Education Manager with the Canadian Donation and Transplantation Research Program. In this role, Manuel builds relationships patient partners with investigators and strengthens capacity among CDTRP patient partners.
ity of Alberta and has received training in patient-oriented research through the University of Calgary’s PACER (Patient and Community Engagement Research) Program. He is the Patient, Family, and Donor Partnerships & Education Manager with the Canadian Donation and Transplantation Research Program. In this role, Manuel builds relationships patient partners with investigators and strengthens capacity among CDTRP patient partners.
 Anders Billström
Anders Billström  Gareth Wiltshire
Gareth Wiltshire
































 K
K
 Sherrie Logan
Sherrie Logan 


 born and raised in Edmonton, AB. With a degree in Music Performance (saxophone performance) and Education, Lindsey has taught for over a decade in the Edmonton Public School system. When she is not teaching, she is very involved in the music community, performing in the Edmonton Winds, conducting her own community ensemble (The Beer League Band) and on numerous musical boards across the province. For the past 5 years, Lindsey has had to take a break from teaching after having her amazing son, George. Maternity leave did not goes as expected as George was diagnosed with Dilated Cardiomyopathy at 5 months old and required 2 heart transplants before the age of 4. During this time, Lindsey has also began to dedicate her time building and expanding her charity: Big Gifts for Little Lives, where they raise money to fund Pediatric Heart Transplant research at the Stollery.
born and raised in Edmonton, AB. With a degree in Music Performance (saxophone performance) and Education, Lindsey has taught for over a decade in the Edmonton Public School system. When she is not teaching, she is very involved in the music community, performing in the Edmonton Winds, conducting her own community ensemble (The Beer League Band) and on numerous musical boards across the province. For the past 5 years, Lindsey has had to take a break from teaching after having her amazing son, George. Maternity leave did not goes as expected as George was diagnosed with Dilated Cardiomyopathy at 5 months old and required 2 heart transplants before the age of 4. During this time, Lindsey has also began to dedicate her time building and expanding her charity: Big Gifts for Little Lives, where they raise money to fund Pediatric Heart Transplant research at the Stollery.













































 Dr. Caigan Du is a scientist at the Vancouver Coastal Health Research Institute and an assistant professor in the Department of Urologic Sciences at the University of British Columbia. He received a Ph.D. degree in Biochemistry in UK and postdoctoral training in Immunology in USA. He is interested in the pathogenesis of kidney ischemia-reperfusion injury and transplant rejection, and molecular control of urinary malignancies. He has been studying the impact of kidney donor-derived factors on renal allograft rejection, and the molecular pathways of kidney injury and regeneration in experimental models. He is also interested in developing medical solution including drugs made from natural compounds for all kinds of health problems, including immune disorders, organ preservation, kidney failure and urinary cancer. He is the PI of many grant supports from the Kidney Foundation of Canada and the Canadian Institutes of Health Research.
Dr. Caigan Du is a scientist at the Vancouver Coastal Health Research Institute and an assistant professor in the Department of Urologic Sciences at the University of British Columbia. He received a Ph.D. degree in Biochemistry in UK and postdoctoral training in Immunology in USA. He is interested in the pathogenesis of kidney ischemia-reperfusion injury and transplant rejection, and molecular control of urinary malignancies. He has been studying the impact of kidney donor-derived factors on renal allograft rejection, and the molecular pathways of kidney injury and regeneration in experimental models. He is also interested in developing medical solution including drugs made from natural compounds for all kinds of health problems, including immune disorders, organ preservation, kidney failure and urinary cancer. He is the PI of many grant supports from the Kidney Foundation of Canada and the Canadian Institutes of Health Research.















 Heather Badenoch is a non-directed living liver donor and communications strategist. As the president of Village PR, she provides strategic communications direction and training to not-for-profit clients in community and health. An active transplant volunteer, Heather helps transplant candidates find living donors by running their public appeals, small and large, pro-bono. She also mentors potential living donors on the path to living donation. Heather is a volunteer with the UHN Centre for Living Organ Donation and the Canadian Donation and Transplant Research Program. She and her spouse adopt rescue dogs and volunteer together with Community Veterinary Outreach, a group providing free veterinary care to the pets of people who are homeless.
Heather Badenoch is a non-directed living liver donor and communications strategist. As the president of Village PR, she provides strategic communications direction and training to not-for-profit clients in community and health. An active transplant volunteer, Heather helps transplant candidates find living donors by running their public appeals, small and large, pro-bono. She also mentors potential living donors on the path to living donation. Heather is a volunteer with the UHN Centre for Living Organ Donation and the Canadian Donation and Transplant Research Program. She and her spouse adopt rescue dogs and volunteer together with Community Veterinary Outreach, a group providing free veterinary care to the pets of people who are homeless.







 Sean Dicks is a clinical psychologist who has 20 years’ experience supporting both families of organ donors and transplant recipients. While it is rare for him to have contact with a donor family and recipient linked to the same organ donation-transplantation event, his contact with families who have lived experience with organ donation on the one hand, and patients who have received transplants on the other hand has provided opportunities to explore how their journeys become linked when they attempt to make sense of and find meaning in their respective crises.
Sean Dicks is a clinical psychologist who has 20 years’ experience supporting both families of organ donors and transplant recipients. While it is rare for him to have contact with a donor family and recipient linked to the same organ donation-transplantation event, his contact with families who have lived experience with organ donation on the one hand, and patients who have received transplants on the other hand has provided opportunities to explore how their journeys become linked when they attempt to make sense of and find meaning in their respective crises.
































































 Terri Hansen-Gardiner is a Cree speaking Metis woman from Northern Saskatchewan as well as a 10 year Breast Cancer Survivor. Her tireless spirit and dedication to her community is undeniable. Terri is a cancer survivor who travels around the province to provide assistance, information, and support to Indigenous patients who are trying to access and navigate the cancer care system.
Terri Hansen-Gardiner is a Cree speaking Metis woman from Northern Saskatchewan as well as a 10 year Breast Cancer Survivor. Her tireless spirit and dedication to her community is undeniable. Terri is a cancer survivor who travels around the province to provide assistance, information, and support to Indigenous patients who are trying to access and navigate the cancer care system.















 Dr. Golnaz Karoubi is Assistant Scientist at the Toronto General HospitalResearch Institute and principal investigator in the Latner Thoracic Research Labs. She currently holds an Assistant Professor appointment in the department of Laboratory Medicine and Pathobiology and a cross-appointment in the department of Mechanical and Industrial Engineering at the University of Toronto. Dr. Karoubi received her PhD in Applied Science and Engineering at the University of Toronto and joined the Lung Regenerative Medicine Program in the Department of Clinical Research in Berne University, Switzerland for a post-doctoral research fellowship. She stayed on as a Group Leader in 2008 to direct the basic and transitional science as related to Cancer Stem Cell and Lung Regenerative Medicine in the Department of Biomedical Research at the University of Berne until 2012. In early 2012, she joined the team of Dr. Tom Waddell at the Toronto General Hospital Research Institute as a Senior Scientific Associate and was appointed to Assistant Scientist at the Toronto General Hospital Research Institute (University Health Network) in November 2019 and to Assistant Professor in the Department of Laboratory Medicine and Pathobiology in July 2020.
Dr. Golnaz Karoubi is Assistant Scientist at the Toronto General HospitalResearch Institute and principal investigator in the Latner Thoracic Research Labs. She currently holds an Assistant Professor appointment in the department of Laboratory Medicine and Pathobiology and a cross-appointment in the department of Mechanical and Industrial Engineering at the University of Toronto. Dr. Karoubi received her PhD in Applied Science and Engineering at the University of Toronto and joined the Lung Regenerative Medicine Program in the Department of Clinical Research in Berne University, Switzerland for a post-doctoral research fellowship. She stayed on as a Group Leader in 2008 to direct the basic and transitional science as related to Cancer Stem Cell and Lung Regenerative Medicine in the Department of Biomedical Research at the University of Berne until 2012. In early 2012, she joined the team of Dr. Tom Waddell at the Toronto General Hospital Research Institute as a Senior Scientific Associate and was appointed to Assistant Scientist at the Toronto General Hospital Research Institute (University Health Network) in November 2019 and to Assistant Professor in the Department of Laboratory Medicine and Pathobiology in July 2020. Dr. Haykal graduated from the University of Ottawa Faculty of Medicine in2007 as class valedictorian and silver medalist, and subsequently completed her residency training in Plastic and Reconstructive Surgery at the University of Toronto in 2016. During her residency, she completed a four-year Doctorate of Philosophy (PhD) in tissue engineering, regenerative medicine and immunology with a focus on tracheal reconstruction. She obtained numerous grants and awards including a CIHR Vanier Scholarship. Dr. Haykal then pursued fellowship training in microsurgical reconstruction at the Albany Medical Centre in New York. Dr. Haykal joined the University Health Network and the Toronto General Hospital in 2018. Her clinical focus is on complex oncological reconstruction and microsurgical reconstruction of the breast, head and neck and extremity. She started a lymphedema program in 2019 where she offers microsurgical techniques for the treatment of lymphedema.
Dr. Haykal graduated from the University of Ottawa Faculty of Medicine in2007 as class valedictorian and silver medalist, and subsequently completed her residency training in Plastic and Reconstructive Surgery at the University of Toronto in 2016. During her residency, she completed a four-year Doctorate of Philosophy (PhD) in tissue engineering, regenerative medicine and immunology with a focus on tracheal reconstruction. She obtained numerous grants and awards including a CIHR Vanier Scholarship. Dr. Haykal then pursued fellowship training in microsurgical reconstruction at the Albany Medical Centre in New York. Dr. Haykal joined the University Health Network and the Toronto General Hospital in 2018. Her clinical focus is on complex oncological reconstruction and microsurgical reconstruction of the breast, head and neck and extremity. She started a lymphedema program in 2019 where she offers microsurgical techniques for the treatment of lymphedema. Dr. Ahmed is a Professor in the Cumming School of Medicine at the University of Calgary. The recipient of the 2022 Hypertension Canada Senior Investigator Award, she is a nephrologist and clinician-scientist with a focus on sex and gender differences in human cardiovascular/kidney physiology and clinical outcomes. Dr. Ahmed is the Vice Chair (Research) for the Department of Medicine, Lead of the Libin Institute Women’s Cardiovascular Health Research Initiative at the University of Calgary and Lead of the Alberta Strategy for Patient Oriented Research Capacity Development Platform. Dr. Ahmed is an Advisory Board member for the Canadian Institutes of Health Research Institute of Gender and Health, a member of the Canadian Medical Association Journal Governing Council and the President-Elect for the Organization for the Study of Sex Differences.
Dr. Ahmed is a Professor in the Cumming School of Medicine at the University of Calgary. The recipient of the 2022 Hypertension Canada Senior Investigator Award, she is a nephrologist and clinician-scientist with a focus on sex and gender differences in human cardiovascular/kidney physiology and clinical outcomes. Dr. Ahmed is the Vice Chair (Research) for the Department of Medicine, Lead of the Libin Institute Women’s Cardiovascular Health Research Initiative at the University of Calgary and Lead of the Alberta Strategy for Patient Oriented Research Capacity Development Platform. Dr. Ahmed is an Advisory Board member for the Canadian Institutes of Health Research Institute of Gender and Health, a member of the Canadian Medical Association Journal Governing Council and the President-Elect for the Organization for the Study of Sex Differences.
















 r. Christopher Nguan is an Associate Professor at the University of British Columbia in the Department of Urological Sciences, Surgical Head of Kidney Transplantation at Vancouver General Hospital and the Director of the Surgical Technologies Experimental Laboratory & Advanced Robotics (STELLAR) facility at Vancouver General Hospital.
r. Christopher Nguan is an Associate Professor at the University of British Columbia in the Department of Urological Sciences, Surgical Head of Kidney Transplantation at Vancouver General Hospital and the Director of the Surgical Technologies Experimental Laboratory & Advanced Robotics (STELLAR) facility at Vancouver General Hospital. r Caroline Lamarche is a clinician scientist and transplant nephrologist at Maisonneuve-Rosemont Hospital. She is an assistant clinical professor at the Université de Montréal. After her nephrology training at the Université de Montréal (2015), she completed a Master degree on the use of adoptive immunotherapy to treat/prevent BK nephropathy in kidney transplant recipients. She then pursued a post-doctoral fellowship with Dr. Megan Levings at the University of British Columbia on the use of chimeric antigen receptor (CAR) regulatory T cells (Tregs) to induce transplant tolerance. Her lab is working on the development of adoptive immunotherapy in nephrology and understanding Treg dysfunction.
r Caroline Lamarche is a clinician scientist and transplant nephrologist at Maisonneuve-Rosemont Hospital. She is an assistant clinical professor at the Université de Montréal. After her nephrology training at the Université de Montréal (2015), she completed a Master degree on the use of adoptive immunotherapy to treat/prevent BK nephropathy in kidney transplant recipients. She then pursued a post-doctoral fellowship with Dr. Megan Levings at the University of British Columbia on the use of chimeric antigen receptor (CAR) regulatory T cells (Tregs) to induce transplant tolerance. Her lab is working on the development of adoptive immunotherapy in nephrology and understanding Treg dysfunction.
















 Dr. Tandon is an Associate Professor of Medicine, Co-Director of the Cirrhosis Care Clinic, Transplant Hepatologist and lead of the Cirrhosis Care Alberta quality improvement program. She obtained her training at the University of Alberta, the Hospital Clinic in Barcelona and Yale University. Her clinical practice and research are focused on cirrhosis with research interests including cirrhosis related complications, malnutrition, frailty, exercise therapy, palliative care, integrative health approaches such as meditation and knowledge translation. It is her career goal to provide wholistic, interdisciplinary, evidence based, patient-centered care through education, empowerment, engagement and team-work.
Dr. Tandon is an Associate Professor of Medicine, Co-Director of the Cirrhosis Care Clinic, Transplant Hepatologist and lead of the Cirrhosis Care Alberta quality improvement program. She obtained her training at the University of Alberta, the Hospital Clinic in Barcelona and Yale University. Her clinical practice and research are focused on cirrhosis with research interests including cirrhosis related complications, malnutrition, frailty, exercise therapy, palliative care, integrative health approaches such as meditation and knowledge translation. It is her career goal to provide wholistic, interdisciplinary, evidence based, patient-centered care through education, empowerment, engagement and team-work. Emily is a PhD student at the University of Alberta, where she completed her Master of Science (MSc) degree in medicine. With a passion for patient-oriented research and a commitment to improving health outcomes, Emily’s doctoral project focuses on EMPOWER, a 12-week online mind-body wellness program designed for adults living with chronic health conditions.
Emily is a PhD student at the University of Alberta, where she completed her Master of Science (MSc) degree in medicine. With a passion for patient-oriented research and a commitment to improving health outcomes, Emily’s doctoral project focuses on EMPOWER, a 12-week online mind-body wellness program designed for adults living with chronic health conditions. Dr Basil S. Nasir, M.B.B.Ch
Dr Basil S. Nasir, M.B.B.Ch Dr. Victor Ferreira is an Assistant Scientist in the Ajmera Transplant Centre at the University Health Network (UHN) and the Toronto General Hospital Research Institute (TGHRI). He is also an Assistant Professor at the University of Toronto in the Department of Laboratory Medicine and Pathobiology (LMP). He completed his PhD in Medical Sciences – Infection and Immunity Specialization at McMaster University in 2014 and post-doctoral training at UHN. His research program is focused on three pillars: I) using systems vaccinology to reveal insights into vaccine responses in immunocompromised individuals like transplant recipients; II) characterizing the impact of chronic viral infection on host immune responses; and III) developing novel methods for eliminating latent viruses in human organs. His work has been cited >2,500 times and is published in journals including the New England Journal of Medicine, Nature Immunology, the Lancet Infectious Diseases, Clinical Infectious Diseases, the American Journal of Transplantation and Journal of Infectious Diseases.
Dr. Victor Ferreira is an Assistant Scientist in the Ajmera Transplant Centre at the University Health Network (UHN) and the Toronto General Hospital Research Institute (TGHRI). He is also an Assistant Professor at the University of Toronto in the Department of Laboratory Medicine and Pathobiology (LMP). He completed his PhD in Medical Sciences – Infection and Immunity Specialization at McMaster University in 2014 and post-doctoral training at UHN. His research program is focused on three pillars: I) using systems vaccinology to reveal insights into vaccine responses in immunocompromised individuals like transplant recipients; II) characterizing the impact of chronic viral infection on host immune responses; and III) developing novel methods for eliminating latent viruses in human organs. His work has been cited >2,500 times and is published in journals including the New England Journal of Medicine, Nature Immunology, the Lancet Infectious Diseases, Clinical Infectious Diseases, the American Journal of Transplantation and Journal of Infectious Diseases.